Isolation and Antibiotic Susceptibility Pattern of MRSA among Staphylococcus Aureus Causing Wound Infection
Abstract
Introduction: Staphylococcus aureusis one of the most common cause of wound infection. Emergence of Methicillin Resistant Staphylococcus aureus (MRSA) is associated with failure of treatment resulting in prolonged illness, higher healthcare expenditure and increased mortality.
Aims and Objectives: 1) To isolate and determine antibiotic susceptibility pattern of Staphylococcus aureusisolates causing wound infections. 2) To identify the Methicillin Resistant Staphylococcus aureus(MRSA) strains among the isolates and to detect the presence of Vancomycin resistance among them.
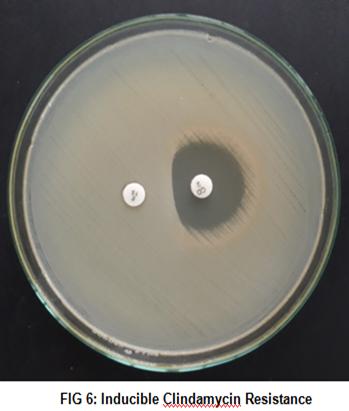
Materials and Methods: 100 isolates of Staphylococcus aureuswere identified over a period of 3 months, from wound swab samples, for which antimicrobial susceptibility test was performed by Kirby-Bauer disc diffusion method. The Staphylococcus aureusisolates were tested for MRSA, by Cefoxitin Disc Diffusion Method. MRSA isolateswere tested for Inducible Clindamycin resistance by D test and Vancomycin susceptibility by E-Test.
Results: From 434 wound swab samples, 100 isolates of Staphylococcus aureuswere identified accounting for 25.91%. The prevalence of MRSA was found to be 24%. All the isolates of MRSA were sensitive to Linezolid and Tigecycline. Of the 16 isolates with resistance to erythromycin, 4 isolates showed constitutive clindamycin resistance(25%) and 9 showed inducible clindamycin resistance(56.25%). All the MRSA isolates were found to be susceptible to vancomycin with MIC <2µg/ml.
Conclusion: Routine screening for MRSA and their antibiotic susceptibility pattern is crucial for monitoring infection control practices. Periodic studies on drug resistance patterns is the need of the hour for establishing antimicrobial stewardship with a view towards improving patient care.
Keywords: Staphylococcus aureus, wound infection, Methicillin Resistant Staphylococcus aureus(MRSA), Inducible clindamycin resistance.
Full Text:
PDFReferences
NivedithaNagasundaram, SujathaSistla. Is There a Need to Revise the Antibiotic Concentration in Clinical and Laboratory Standards Institute-Recommended Oxacillin Screen Agar? IJMM (2017); 35(2): 243-6
Derek F. J. Brown, David I. Edwards, Peter M. Hawkey. Guidelines for the laboratory diagnosis and susceptibility testing of methicillin-resistant Staphylococcus aureus (MRSA). Journal of Antimicrobial Chemotherapy (2005); 56: 1000–18
K Rajaduraipandi, KR Mani, K Panneerselvam, M Mani, M Bhaskar, P Manikandan. Prevalence and Antimicrobial Susceptibility Pattern of Methicillin Resistant Staphylococcus aureus: A Multicentre Study. IJMM (2006);24(1):34-8
Ravichandran B, Thyagarajan Ravinder, Radhika Katragadda, K.V.Leela. Bacterial Profiling of isolated MRSA strains from wounds in tertiary care hospital, Chennai, Tamilnadu, India. IPP (2015); 3(2): 580-5
P Kale, B Dhawan. The changing face of community- acquired methicillin-resistant Staphylococcus aureus. IJMM (2016); 34(3): 275 285
Stefani S, Chung DR, Lindsay JA, Friedrich AW, Kearns AM, Westh H, et al. Methicillin-resistant Staphylococcus aureus (MRSA): Global epidemiology and harmonisation of typing methods. Int J Antimicrob Agents (2012);39: 273-82.
David MZ, Daum RS. Community-associated methicillin resistant Staphylococcus aureus: Epidemiology and clinical consequences of an emerging epidemic. ClinMicrobiol Rev 2010; 23: 616-87
Indian Network for Surveillance of Antimicrobial Resistance (INSAR) group, India. Methicillin resistant Staphylococcus aureus (MRSA) in India: Prevalence & susceptibility pattern. Indian J Med Res (2013);137:363-
Datta P, Gulati N, Singla N, Vasdeva HR, Bala K, Chander J, et al. Evaluation of various methods for the detection of methicillin resistant Staphylococcus aureus strains and susceptibility patterns. J Med Microbiol. (2011);60:1613–16
Inomata S, Yano H, Tokuda K, Kanamori H, Endo S, Ishizawa C, et al. Microbiological and molecular epidemiological analyses of community-associated methicillin — resistant Staphylococcus aureus at a tertiary care hospital in Japan.J Infect Chemother(2015);21:729-36.
Karsten Becker, Olivier Denis, Sandrine Roisin. Detection of mecA- and mecC-Positive Methicillin-Resistant Staphylococcus aureus (MRSA) Isolates by the New Xpert MRSA Gen 3 PCR Assay. JCM Jan 2016;54 (1):181-4
Koneman’s Textbook of Diagnostic Microbiology 7th edition.
JB Sarma, GU Ahmed, Characterisation of methicillin resistant S. aureus strains and risk factors for acquisition in a teaching hospital in northeast India. IJMM (2010); 28(2): 127-9
S Srinivasan, D Sheela, Shashikala, R Mathew, J Bazroy, R Kanungo.Risk Factors and Associated Problems in the Management of Infections with Methicillin Resistant Staphylococcus aureus. IJMM (2006); 24 (3): 182-5
A S Haddadin, S A Fappiano, P A Lipsett. Methicillin resistant Staphylococcus aureus (MRSA) in the intensive care unit. Postgrad Med J (2002);78:385–392
Egea AL, Gagetti P, Lamberghini R, Faccone D, Lucero C, Vindel A, et al. New patterns of methicillin resistant Staphylococcus aureus (MRSA) clones, community associated MRSA genotypes behave like healthcare-associated MRSA genotypes within hospitals, Argentina. Int J Med Microbiol(2014);304:1086-99.
Kali A, Stephen S, Umadevi S, Kumar S, Joseph NM, Srirangaraj S. Changing trends in resistance pattern of methicillin resistant Staphylococcus aureus. J ClinDiagn Res(2013);7:1979-82
In-GyuBae, Jerome J. Federspiel,Jose´M. Miro. Heterogeneous Vancomycin-Intermediate Susceptibility Phenotype in Bloodstream Methicillin-Resistant Staphylococcus aureus Isolates from an International Cohort of Patients with Infective Endocarditis: Prevalence, Genotype and Clinical Significance.JID(2009);200:1355-66
Susan S. Huang, Sheryl L. Rifas-Shiman, David K. Warren,Victoria J. Fraser, Michael W. Climo. Improving Methicillin-Resistant Staphylococcus aureus, Surveillance and Reporting in Intensive Care Units. JID (2007);195 (1): 330-8.
Sowndarya Visalachy, Kennedy Kumar Palraj, Sridharan Sathya Moorthy Kopula, Uma Sekar. Carriage of Multidrug Resistant Bacteria on Frequently Contacted Surfaces and Hands of Health Care Workers. JCDR (2016); 10(5): 18-20
Rakesh Kumar Panda, AshokaMahapatra, Bandana Mallick. Evaluation of Genotypic and Phenotypic Methods for Detection of Methicillin Resistant Staphylococcus aureus in a Tertiary Care Hospital of Eastern Odisha. JCDR.(2016) ;10(2): 19-21
Kiran K. Mokta, Santwana Verma, Divya Chauhan. Inducible Clindamycin Resistance among Clinical Isolates of Staphylococcus aureus from Sub Himalayan Region of India. JCDR (2015); 9(8): 20-3
Susmita Bhattacharya, Kuhu Pal, Sonia Jain, Shiv SekharChatterjee. Surgical Site Infection by Methicillin Resistant Staphylococcus aureus– on Decline? JCDR (2016); 10(9): 32-36
Sunanda Rajkumar, Sujatha Sistla, Meerabai Manoharan, Madhan Sugumar, Niveditha Nagasundaram, Subhash Chandra Parija, Pallab Ray, Yamuna Devi. Prevalence and Genetic Mechanisms of Antimicrobial Resistance in Staphylococcus Species: A Multicentre Report of the Indian Council of Medical Research Antimicrobial Resistance Surveillance Network. IJMM (2017);35(1):53-9
Kailash Moolchandani, Apurba Sankar Sastry, Deepa Shree, Sujatha Sistla, Harish. Antimicrobial Resistance Surveillance among Intensive Care Units of a Tertiary Care Hospital in Southern India. JCDR (2017); 11(2): 1-7
Harsh Kumar, Amritbeer Kaur, Nitin Kishore. Prevalence of Multiple Antibiotic Resistant Nasal Carriage MRSA Among Healthy Population of Border Villages in Amritsar Region, Punjab, India. JCDR (2016); 10(5): 1-2
S Vidhani, PL Mehndiratta, MD Mathur. Study of Methicillin Resistant S. Aureus (MRSA) Isolates from High Risk Patients. IJMM (2001); 19(2): 13-6
Vikrant Negi, Shekhar Pal, Deepak Juyal. Bacteriological Profile of Surgical Site Infections and Their Antibiogram: A Study from Resource Constrained Rural Setting of Uttarakhand State, India. JCDR (2015); 9(10):17-20
James S. Lewis and James H. Jorgensen. Inducible Clindamycin Resistance in Staphylococci: Should Clinicians and Microbiologists be Concerned? Antimicrobial Resistance. CID (2005); 40 :280-5
SoumyadeepGhosh,Mandira Banerjee. Methicillin resistance & inducible clindamycin resistance in Staphylococcus aureus. Ind J Med Res. (2016);143: 362-6
B. Sasirekha, M. S. Usha, J. A. Amruta. Incidence of constitutive and inducible clindamycin resistance among hospital-associated Staphylococcus aureus. Biotech (2014) 4:85–9
Arjun OjhaKshetry, Narayan Dutt Pant, Raju Bhandari, Sabita Khatri. Minimum inhibitory concentration of vancomycin to methicillin resistant Staphylococcus aureus isolated from different clinical samples at a tertiary care hospital in Nepal. Antimicrobial Resistance and Infection Control (2016); 5(27):1-6
Refbacks
- There are currently no refbacks.

This work is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License.
An initiative of The Tamil Nadu Dr M.G.R. Medical University